Clinical Trials
NPR’s Talk of the Nation Science Friday
Meet AIRM’s Chief Scientific Officer: Dr. Robert LanzaRegenerative medicine could revolutionize medicine, and provide therapies for the world’s most deadly conditions...

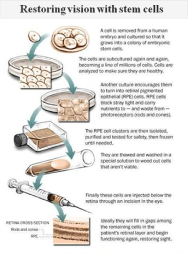
Front Page, Washington Post: Embryonic Stem Cells Appear to Restore Vision to Legally Blind Patient
For the first time, an experimental treatment made from human embryonic stem cells has shown evidence of helping someone, partially restoring sight to two people suffering from slowly progressing forms of blindness By Rob Stein and David Brown For the first time, an...
Reuters: First patients shown to improve with embryonic stem cells
The first-ever report of the medical use of stem cells taken from human embryos
TIME: Early Success in a Human Embryonic Stem Cell Trial to Treat Blindness
The first patients to receive human embryonic stem cell transplants say their lives have been transformed by the experimental procedure By Alice Park | @aliceparkny | January 24, 2012 | For the researchers at UCLA’s Jules Stein Eye Institute who launched the first-ever study to show...

CBSNEWS: Stem Cell treatment could aid leading cause of blindness
Possible stem cell treatment for Macular Degeneration, the leading cause of blindness in older Americans
The Guardian: First Trial of Embryonic Stem Cell Treatment in Europe Gets Green Light
“This is the first time an embryonic stem cell therapy has been approved in Europe,” said Lanza. Retinal cells derived from embryonic stem cells will be injected behind patients’ retinas.Photograph: Roger Tooth/Guardian British surgeons are to take part in the...
Washington Post: First patients treated in new human embryonic stem cell study
“The initiation of these two clinical trials marks an important turning point for the field.” said Robert Lanza, the company’s chief scientific officer. Researchers at UCLA treat the first patients in the second FDA-approved study evaluating a therapy made from...
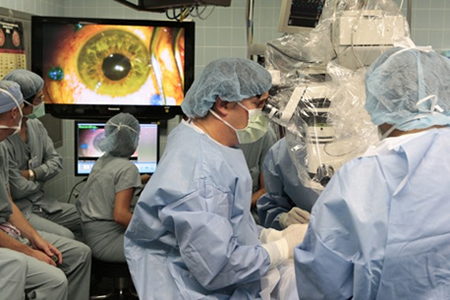
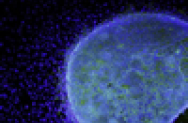
MIT Technology Review: Two Patients Undergo Stem-Cell Blindness Treatment
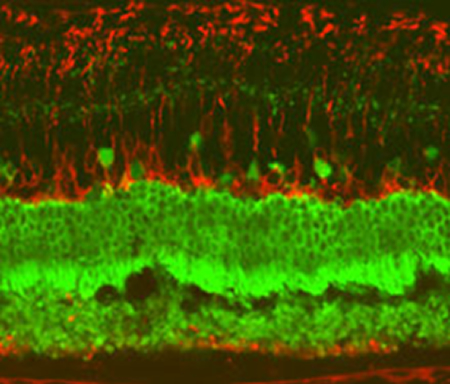
“Researchers see the start of a second set of tests, in blindness, as an important landmark for the stem-cell field.” Eye opener: A confocal microscope image of a rat retina after injection of human retinal pigment epithelium cells.Credit: Advanced Cell Technologies...
TIME: The Tiniest Transplant
The great promise of stem cells is finally being put to the test. “We are finally ready to break ground on this field with the first trials,” says Dr. Robert Lanza, “It’s taken a decade of extensive research to get...

NEWSWEEK: Saving Sight, Testing Faith
Lanza’s dream of turning human embryonic stem cells into therapies for the sick and the suffering is taking a huge step closer to reality. May 23 & 30, 2011 Stem cells from embryos may finally cure patients—reviving a bitter debate....

USA Today: Embryonic stem cell trial to test macular degeneration treatment
Federal officials have approved the start of human embryonic stem cell treatment experiments on patients suffering a leading cause of vision loss.

TIME: FDA Approves Second Trial of Stem-Cell Therapy
For only the second time, the Food and Drug Administration approved a company’s request to test an embryonic stem cell-based therapy on human patients.
Reuters: Second U.S. Company Gets Stem Cell Go-Ahead
The U.S. Food and Drug Administration has approved the second human trial of human embryonic stem cells — this one testing cells in people with a progressive form of blindness.
BBC: Human Trials to Start for Stem Cell Treatment for Blindness
An American biotech company has just announced that it has been licensed to begin human trials of a stem cell treatment for blindness.

Washington Post: New Embryonic Stem Cell Experiment
Government regulators have given the go-ahead to a second study that will for the first time carefully test a treatment created using human embryonic stem cells in people, according to the company sponsoring the experiment.

USA Today: Second human embryonic stem cell clinical trial to start
Federal officials have cleared a second clinical trial of a human embryonic stem-cell treatment, a company announced Monday, for a progressive blindness syndrome affecting young people.

Associated Press: Feds OK 2nd Human Study of Embryonic Stem Cells
NEW YORK (AP) – For only the second time, the U.S. government has approved a test in people of a treatment using embryonic stem cells – this time for a rare disease that causes serious vision loss.
New York Times: 2 Treatments for Retinas Make Gains
Advanced Cell Technology, of Marlborough, Mass., said it would test its stem cell therapy on 12 adults with severe vision loss caused by Stargardt’s, an inherited disease. The company has turned human embryonic stem cells into retinal pigment epithelial cells,...

Paradigm Shifter: An Interview with Robert Lanza
“An interview with the author of Biocentrism, a book that Nyogen Roshi (the last authorized disciple of Taizan Maezumi Roshi, the foremost Zen master of the twentieth century) describes as mirroring his experiences in the practice of zazen as closely...
